 Cu Resin is used for the selective separation of copper metal ions, such as the nuclear medicine isotopes Cu-64 and Cu-67. The uptake on Cu Resin of metal ions representative of Zinc or enriched Nickel targets have been determined from various acid matrices.
Cu Resin is used for the selective separation of copper metal ions, such as the nuclear medicine isotopes Cu-64 and Cu-67. The uptake on Cu Resin of metal ions representative of Zinc or enriched Nickel targets have been determined from various acid matrices.
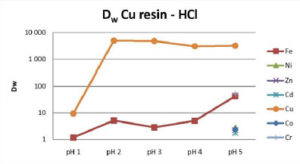
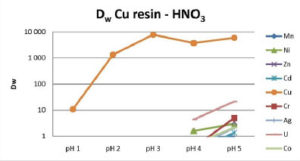 Cu Resin selectively retains copper from pH 2 to 5 for HCl, HNO3 and H2SO4 over all the tested cations, including Ni and Zn. In HCl and H2SO4 media, iron may also be partially coextracted. The Cu/Fe selectivity decreases as pH increases. The Cu/Fe separation factor is about 1000 at pH 2 and about 70 at pH 5 (Fig 1-3).
Cu Resin selectively retains copper from pH 2 to 5 for HCl, HNO3 and H2SO4 over all the tested cations, including Ni and Zn. In HCl and H2SO4 media, iron may also be partially coextracted. The Cu/Fe selectivity decreases as pH increases. The Cu/Fe separation factor is about 1000 at pH 2 and about 70 at pH 5 (Fig 1-3).
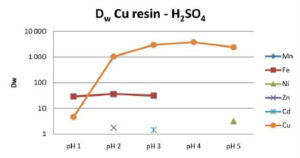 Cu uptake is generally high at pH values greater than 2, while Cu can be easily eluted with mineral acids of concentrations greater than 0.1 moles/L.
Cu uptake is generally high at pH values greater than 2, while Cu can be easily eluted with mineral acids of concentrations greater than 0.1 moles/L.
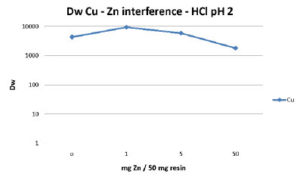
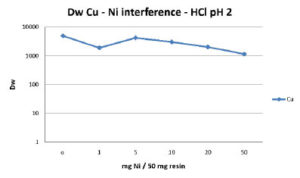
The main application of the Cu resin is the separation of Cu-64 or Cu-67 from irradiated Zn or Ni targets. Figures 4 and 5 summarize the influence of Zn and Ni on the extraction of Cu. High amounts of both elements interfere only slightly with the Cu uptake in HCl at pH 2. Even at loadings of 1g of Ni or Zn per g of Cu resin the Dw(Cu) remains greater than 1000.
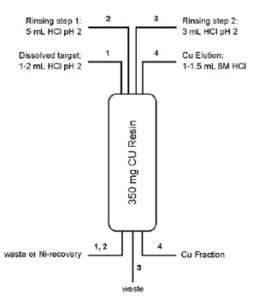 A method for the separation of Cu from Ni and Zn targets was optimized using simulated target solutions (1). Two types of solutions were tested:
A method for the separation of Cu from Ni and Zn targets was optimized using simulated target solutions (1). Two types of solutions were tested:
1) simulated Ni target solutions containing 10 μg each of Cu, Co, Zn and 200 mg of Ni in 5 mL HCl at pH 2, and 2) simulated Zn target solutions containing 10 μg each of Cu, Co, Ni and 200 mg Zn in 5 mL HCl at pH 2.
For both simulated target solutions, Ni, Zn and Co are quantitatively removed from the column during sample solution loading and rinsing whereas Cu is recovered in high yield (>85%) in 1 – 1.5 mL 8M HCl (2,3).
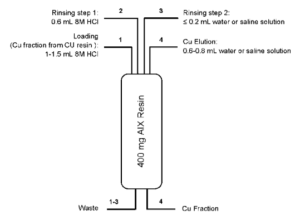
Further optimization of the elution conditions led to the method shown in Figure 6 (2).
The Cu separation method can be performed at elevated flow rates (e.g. using a vacuum box) without impacting its performance. Loading of the column and Cu elution should be done at ~1 mL/min. Rinsing of the column can be done at up to 6 mL/min, and the entire Cu separation can thus be obtained in 3 – 5 minutes.
Overall decontamination factors obtained are high (Table 1). Cu yields were found to be in the order of 90% in 1 mL of 8M HCl and > 95% in 1.5 mL 8M HCl.
| Element | Decontamination Factor |
| Ni | > 20,000 |
| An | > 40,000 |
| Ga | > 10,000 |
| Co | > 30,000 |
| Au | > 30,000 |
For certain applications the Cu eluate might be too acidic. In these cases, it is possible to reduce the acidity of the Cu eluate using a small anion exchange column. Fig. 7 schematically shows such a conversion method using anion exchange resin (AIX). In addition to converting the Cu eluate from high acid conditions to low acid or neutral conditions the conversion step also further concentrates the Cu and increases Ni, Zn, Au and organic impurity decontamination.
Cu Resin is manufactured in one particle size 100-150μm and is only available in bottles at this time. Slurry packing the resin can be difficult due to its relatively high hydrophobicity. For guidance on packing Cu resin, please see Eichrom application note AN-1703.
| Particle Size | Bottles | Part Number |
| 100-150 μm | 25 grams | CU-B25-A |
| 50 grams | CU-B50-A | |
| 100 grams | CU-B100-A | |
| 200 grams | CU-B200-A |