 Eichrom’s RE Resin is an extraction chromatographic material which contains the same extractant as TRU Resin, octyl(phenyl)-N,N-diisobutylcarbamoyl-methylphosphine oxide (abbreviated as CMPO)in tributyl phosphate(TBP) coated on an inert methacrylic polymeric support. The CMPO molecule is shown in Figure 1. RE Resin contains a higher concentration of CMPO than TRU Resin to improve the retention of trivalent rare earths, actinides and Fe(III).
Eichrom’s RE Resin is an extraction chromatographic material which contains the same extractant as TRU Resin, octyl(phenyl)-N,N-diisobutylcarbamoyl-methylphosphine oxide (abbreviated as CMPO)in tributyl phosphate(TBP) coated on an inert methacrylic polymeric support. The CMPO molecule is shown in Figure 1. RE Resin contains a higher concentration of CMPO than TRU Resin to improve the retention of trivalent rare earths, actinides and Fe(III).
RE Resin is a favorable tool for the group separation of rare earth elements and has been used in conjunction with geological dating and radionuclide transport studies. Due to the high retention of yttrium on RE Resin it has also been applied to the purification of yttrium (Y90) used in cancer therapy. The DGA resins have replaced RE and TRU in many applications. However, RE and TRU still offer unique selectivity that may offer advantages over DGA in some applications.
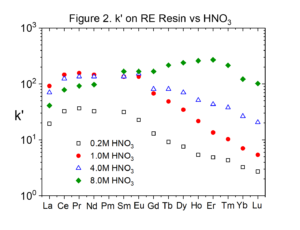
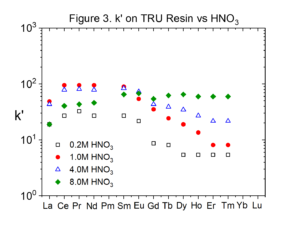
Figures 2 and 3 compare the uptake of selected lanthanide elements on RE and TRU Resins from HNO3 (Data replotted from Huff and Huff HD193). The uptake of the rare earths is typically twice as high on RE Resin than on TRU Resin.
Experiments conducted by Huff & Huff show that the only transition metals coextracted with rare earths and actinides from HNO3 by RE and TRU are Zr(IV) and Fe(III).
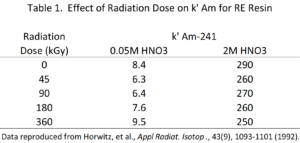
Dietz and Horwitz (1992) investigated the use of RE Resin in the production of 90Y for radiopharmaceutical use. In this application, the radiation stability of the resin is quite important because of the high activities loaded onto the column. RE Resin was exposed to increasing doses of absorbed radiation, and the k’ for americium from low acid (0.05M HNO3) and high acid (2M HNO3) concentrations was measured. The data indicates that the RE Resin was not significantly affected by the radiation exposure of up to 360 gGy (Table 1).
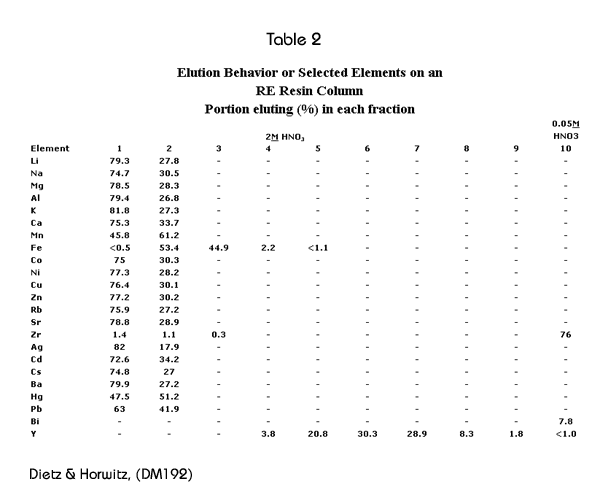
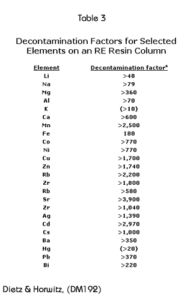 Table 2 shows the elution behavior of various elements on the RE Resin. Fractions 1-6 correspond to 1.8 FCV each, fractions 7 and 8 to 3.6 FCV each, and fractions 9 and 10 to 9.0 FCV each. This data indicates that elements which are commonly present in environmental samples can be readily separated from rare earth elements using RE Resin.
Table 2 shows the elution behavior of various elements on the RE Resin. Fractions 1-6 correspond to 1.8 FCV each, fractions 7 and 8 to 3.6 FCV each, and fractions 9 and 10 to 9.0 FCV each. This data indicates that elements which are commonly present in environmental samples can be readily separated from rare earth elements using RE Resin.
Decontamination factors for the elements shown in Table 2 were calculated by dividing the initial concentration of that element by the concentration found in fraction number 5. These are presented in Table 3. Numbers presented as “>” were calculated using the detection limit in the denominator. It can be seen, that generally, these decontamination factors are quite large.
RE Resin is manufactured in three particle sizes (20-50 µm, 50-100 µm, and 100-150 µm) and is sold in bottles or ready to use in prepackaged columns (for gravity flow) and cartridges (for vacuum assisted flow.) Click here for part numbers and descriptions.
Source: Huff, E.A., and Huff, D.R., “TRU Spec and RE Spec Chromatography: Basic Studies and Applications,” 34th ORNL/DOE Conference On Analytical Chemistry In Energy Technology. Gatlinburg TN, (1993) Eichrom Reference HD193.
Dietz, M.L., Horwitz, E.P., “Improved Chemistry for the Production of Yttrium-90 for Medical Applications,” Appl. Radiat. and Isotop., 43(9), 1093-1101 (1992).