Eichrom’s Sr Resin contains 4,4′(5′)-di-t-butylcyclohexano 18-crown-6 (crown ether) in 1-octanol (Figure 1.) on an inert polymeric support. The bed density of Sr Resin is approximately 0.35 g/mL.
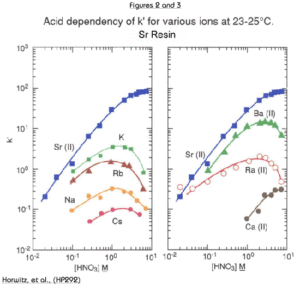
Figures 2 through 5 show the acid dependency of k’for strontium on Sr Resin, plotted with curves for various other elements. Horwitz, et al. reported these data from studies performed with experimental batches of Sr Resin. Eichrom’s commercial product conforms to established specifications that ensure proper performance of Eichrom issued methods. Please refer to our product specifications for details.
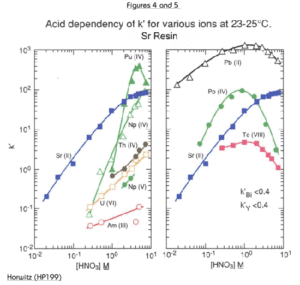 The uptake of strontium by Sr Resin increases with increasing nitric acid concentration. At 8M HNO3, the k’ for Sr is approximately 90. The k’ value for Sr falls to less than 1 at concentrations of HNO3 less than 0.05M. The alkali and alkaline earth metals (Figures 2 and 3) show much lower affinity for the resin than strontium across the concentration range of nitric acid shown. Among the alkaline earth metals, calcium has lowest uptake on Sr Resin. It is, therefore, relatively easy to separate Sr from Ca. Historical methods to accomplish the separation of Sr from the other alkaline earth metal cations are tedious and require multiple precipitations using very hazardous fuming nitric acid. Barium retention is higher than Ca or Ra. However, Ba uptake peaks at about 3M nitric acid and falls off at higher concentrations. To ensure adequate decontamination of Ba in Sr analysis, load Sr on the resin from 8M nitric acid. With adequate rinsing, Ba will be washed from the column leaving a pure Sr fraction.
The uptake of strontium by Sr Resin increases with increasing nitric acid concentration. At 8M HNO3, the k’ for Sr is approximately 90. The k’ value for Sr falls to less than 1 at concentrations of HNO3 less than 0.05M. The alkali and alkaline earth metals (Figures 2 and 3) show much lower affinity for the resin than strontium across the concentration range of nitric acid shown. Among the alkaline earth metals, calcium has lowest uptake on Sr Resin. It is, therefore, relatively easy to separate Sr from Ca. Historical methods to accomplish the separation of Sr from the other alkaline earth metal cations are tedious and require multiple precipitations using very hazardous fuming nitric acid. Barium retention is higher than Ca or Ra. However, Ba uptake peaks at about 3M nitric acid and falls off at higher concentrations. To ensure adequate decontamination of Ba in Sr analysis, load Sr on the resin from 8M nitric acid. With adequate rinsing, Ba will be washed from the column leaving a pure Sr fraction.
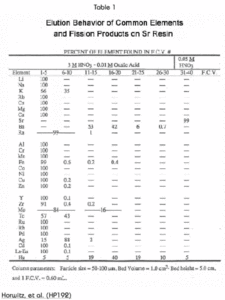 The tetravalent actinides show significant retention on the Sr Resin from high concentrations of HNO3 (Figure 4). The addition of oxalic acid as a competitive complexing agent will prevent the retention of actinide elements on the column. The elution behavior of a selection of elements, in addition to those discussed above, is shown in Table 1.
The tetravalent actinides show significant retention on the Sr Resin from high concentrations of HNO3 (Figure 4). The addition of oxalic acid as a competitive complexing agent will prevent the retention of actinide elements on the column. The elution behavior of a selection of elements, in addition to those discussed above, is shown in Table 1.
The retention of Pb by Sr Resin is high across a broad range of nitric acid concentrations. (Figure 5.) It is difficult to strip Pb from this resin without complexing agents. A modified version of the Sr Resin, containing a lower concentration of the crown ether, has been developed for Pb analysis (Eichrom’s Pb Resin) to facilitate stripping of Pb.
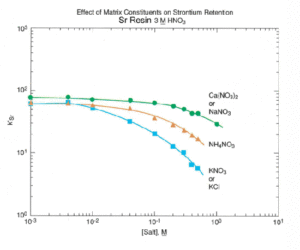
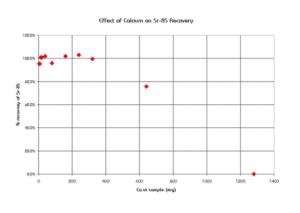 Sr Resin shows resilience to the interference of a number of cations commonly found in environmental and biological samples. Figure 6 shows the effect of various cations on strontium uptake by Sr Resin. Ca and Na cause minimal interference for concentrations of up to 0.5 moles/L. The high levels of calcium found in certain sample types, like milk, can exceed this concentration and affect the recovery of Sr on Sr Resin. In a study conducted at BNFL (formerly Magnox Electric, Berkley, UK), a Sr-85 spike was loaded out of 8M HNO3 onto a preconditioned, 2 mL Sr Resin column. The column was rinsed with 8M HNO3 and Sr was eluted with water. The effect of calcium content in the sample on the recovery of Sr-85 is shown in Figure 7. Recovery was quantitative for calcium levels up to 320 mg using a 2 mL column of Sr Resin. Above that level, however, chemical yields declined.
Sr Resin shows resilience to the interference of a number of cations commonly found in environmental and biological samples. Figure 6 shows the effect of various cations on strontium uptake by Sr Resin. Ca and Na cause minimal interference for concentrations of up to 0.5 moles/L. The high levels of calcium found in certain sample types, like milk, can exceed this concentration and affect the recovery of Sr on Sr Resin. In a study conducted at BNFL (formerly Magnox Electric, Berkley, UK), a Sr-85 spike was loaded out of 8M HNO3 onto a preconditioned, 2 mL Sr Resin column. The column was rinsed with 8M HNO3 and Sr was eluted with water. The effect of calcium content in the sample on the recovery of Sr-85 is shown in Figure 7. Recovery was quantitative for calcium levels up to 320 mg using a 2 mL column of Sr Resin. Above that level, however, chemical yields declined.
Note: In some circumstances, Sr may be recovered from Sr Resin using deionized water. However, for consistent high recovery of Sr, Eichrom recommends using dilute HNO3 (0.05 moles/L) to elute Sr from Sr resin.
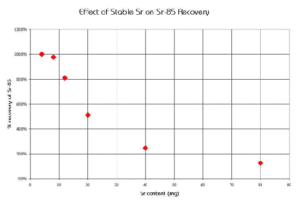
A similar study was conducted to measure the effect of increasing Sr content in a sample on the chemical recovery of Sr-89 on a 2 mL column of Sr Resin (Figure 8). The chemical recoveries were quantitative when loading 4 and 8 mg of Sr, but decreased significantly at higher loadings of Sr.
The experimentally determined maximum capacity of the resin for Sr is approximately 12 mg per 2 mL column (Horwitz, et al., HP292.) However, in the recommended working capacity is 8 mg Sr per 2 mL column or cartridge.
As shown in Figure 6, potassium can cause significant reduction in Sr uptake even at relatively low levels. Additionally, the presence of 40K in the Sr fraction could cause a bias in the 90Sr measurement. For these reasons, in samples with sufficient potassium to be problematic, a precipitation of Sr with calcium phosphate or calcium oxalate is recommended to eliminate the potassium interference.
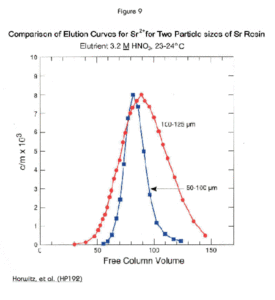
Figure 9 demonstrates the different elution profiles of Sr Resin in two different particle sizes. The smaller particle size produces a narrower the elution band. This means better chromatographic performance, but at the cost of slower gravity flow rates, and, consequently, longer analysis times. Use of vacuum assisted flow permits the use of smaller particle size resins (20-50 µm and 50-100 µm) with faster flow rates than gravity flow would allow. Sr Resin (50-100 µm) is available packed in cartridges for use with Eichrom’s Vacuum Box System.
Sr Resin is manufactured in three particle sizes (20-50 µm 50-100 µm, and 100-150 µm) and is sold in bottles or ready to use in prepackaged columns (for gravity flow) and cartridges (for vacuum assisted flow.) Click here for part numbers and descriptions.