UTEVA Resin has been applied to a variety of analytical challenges, including uranium measurements in environmental samples, removal of uranium from samples prior to analysis for other elements, the sequential determination of uranium, plutonium, and americium, the measurement of actinides in urine, and the measurement of actinides in high level waste.
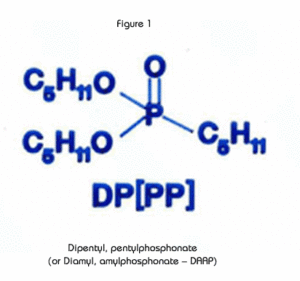
The extractant in the UTEVA Resin, diamyl, amylphosphonate (DAAP, Figure 1), extracts nitrato complexes of the actinide elements. The formation of these complexes is driven by the concentration of nitrate in the sample solution. Therefore, the uptake of the actinides increases with increasing nitric acid concentration. Figure 2 is a plot of the k'(a measure of uptake corresponding to the number of free column volumes to peak maximum) vs. nitric acid concentration.
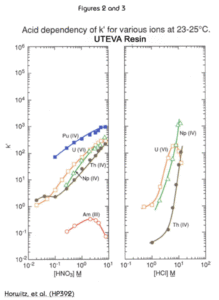 The uptake of each tetravalent actinide and U(VI) on UTEVA from HNO3 is very similar. All have strong retention (k’>100) above 5M nitric acid. Note that Am is not retained at any HNO3 concentration.
The uptake of each tetravalent actinide and U(VI) on UTEVA from HNO3 is very similar. All have strong retention (k’>100) above 5M nitric acid. Note that Am is not retained at any HNO3 concentration.
Figure 3 shows the effect of HCl on the retention of tetravalent neptunium, thorium, and hexavalent uranium on UTEVA Resin. The large difference in k’ for uranium and thorium in the range of 4-6M HCl allows for the selective elution of Th from the resin after both Th and uranium have been loaded from HNO3. To ensure complete elution of Th, oxalic acid is often added to HCl. The oxalic acid forms strong complexes with Th, while having a very limiting effect on the retention of U(VI) on UTEVA.
The data in Figure 2 suggests that uranium can be stripped efficiently from the UTEVA Resin with a relatively small volume of very dilute nitric acid (e.g., 0.01-0.05M). In practice, however, ~1M HCl is more efficient in stripping uranium from UTEVA. Concentrations up to 1M HCl have been shown to quantitatively elute U(VI) in 15 mL from a 2mL pre-packed column or cartridge of UTEVA.
Horwitz, et al. reported the data in Figures 2 and 3 from studies performed with experimental batches of UTEVA Resin. Eichrom’s commercial product conforms to established specifications that ensure proper performance of Eichrom issued methods. Please refer to our product specificationsfor details.
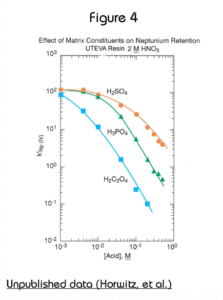
The presence of significant concentrations of matrix elements can affect the performance of methods based on UTEVA Resin. Figures 4 and 5 show the effect of certain polyatomic anions on the retention of neptunium and uranium, respectively, from 2M nitric acid. It should be noted that the effect on tetravalent neptunium is more significant than the affect on uranium. It has been seen in practice that thorium is affected similarly to neptunium by these anions.
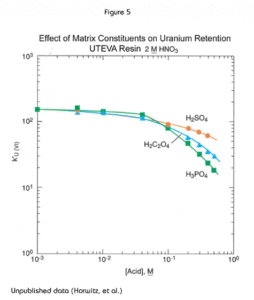 Because phosphate occurs quite commonly in a variety of biological and environmental samples, its effect is most relevant. Fortunately the addition of aluminum to the sample matrix can significantly reduce this issue. Phosphate anion readily complexes tetravalent actinides. This phosphate complex is not extracted by the DAAP. Added aluminum can effectively tie up the phosphate preventing its interference with neptunium (or thorium) uptake by the resin. In some methods, as much as 1M Al(NO3)3 might be added to counteract the effects of phosphate. For samples containing high levels of phosphate, loading from larger volume of Al(NO3)3/HNO3 can improve the recovery of Th(IV) and Np(IV) on UTEVA by diluting the phosphate and providing additional Al to complex phosphate.
Because phosphate occurs quite commonly in a variety of biological and environmental samples, its effect is most relevant. Fortunately the addition of aluminum to the sample matrix can significantly reduce this issue. Phosphate anion readily complexes tetravalent actinides. This phosphate complex is not extracted by the DAAP. Added aluminum can effectively tie up the phosphate preventing its interference with neptunium (or thorium) uptake by the resin. In some methods, as much as 1M Al(NO3)3 might be added to counteract the effects of phosphate. For samples containing high levels of phosphate, loading from larger volume of Al(NO3)3/HNO3 can improve the recovery of Th(IV) and Np(IV) on UTEVA by diluting the phosphate and providing additional Al to complex phosphate.
The theoretical maximum loading capacity of UTEVA Resin is approximately 50 mg of U(VI) per mL of resin bed. In practice, it is not recommended to exceed 20% of this amount, or 10 mg per mL of resin. This corresponds to a working capacity for uranium of 20 mg per 2 mL pre-packed column. The bed density of UTEVA Resin is 0.39 g/mL.
UTEVA Resin is manufactured in three particle sizes (20-50 mm, 50-100 mm, and 100-150 mm) and is sold in bottles or, ready to use, in prepackaged columns (for gravity flow) and cartridges (for vacuum assisted flow.) Click here for part numbers and descriptions.
Source: Horwitz, E.P., et al, Separation and preconcentration of uranium from acidic media by extraction chromatography, Analytica Chimica Acta, Vol.266, pp. 25-37(1992) (HP392)