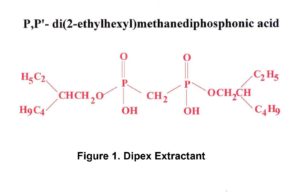
Eichrom’s Actinide Resin is based on the DIPEX® Extractant shown in Figure 1.
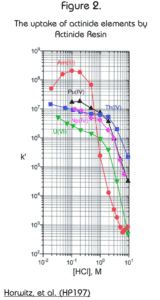 The resin exhibits an extraordinarily high affinity for the actinide elements (Figure 2.) For acid concentrations less than 1M, the retention of actinides is dramatically higher on Actinide Resin than on TRU Resin. This makes the resin quite useful for the preconcentration of actinides out of large volume aqueous samples and for monitoring actinides in aqueous discharges. The resin may be batch contacted with an aqueous sample and then counted directly by liquid scintillation for a very rapid analysis.
The resin exhibits an extraordinarily high affinity for the actinide elements (Figure 2.) For acid concentrations less than 1M, the retention of actinides is dramatically higher on Actinide Resin than on TRU Resin. This makes the resin quite useful for the preconcentration of actinides out of large volume aqueous samples and for monitoring actinides in aqueous discharges. The resin may be batch contacted with an aqueous sample and then counted directly by liquid scintillation for a very rapid analysis.
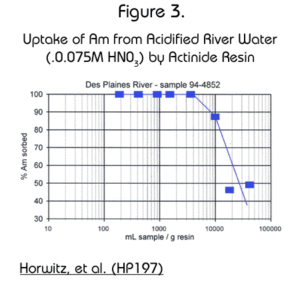
Dr. E. Philip Horwitz, and his group at Argonne National Laboratory, measured the uptake of Am by the Actinide Resin from acidified river water. In their study, the ratio of sample size to amount of Actinide Resin was varied. The results, shown in Figure 3, show that one gram of the resin is able to extract 99% of Am activity from up to 4 liters of water and 90% from up to 10 liters of water.
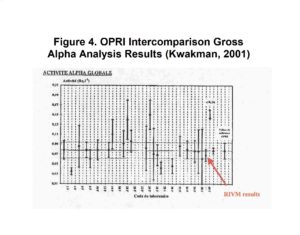
 Additional studies have shown the Actinide Resin to be an excellent choice for preconcentrating elements typically included in gross alpha measurements. (Eichrom method ACW11.) This approach was employed by Pieter Kwakman of RIVM (Netherlands) in a European gross alpha radioactivity in water intercomparison exercise sponsored by OPRI (France.) His method employed a 4 hour batch contact of 0.3g Actinide Resin and 100 mL water sample. LSC counting for 4 hours resulted in a detection limit of 0.03Bq/L (0.8 pCi/L.)
Additional studies have shown the Actinide Resin to be an excellent choice for preconcentrating elements typically included in gross alpha measurements. (Eichrom method ACW11.) This approach was employed by Pieter Kwakman of RIVM (Netherlands) in a European gross alpha radioactivity in water intercomparison exercise sponsored by OPRI (France.) His method employed a 4 hour batch contact of 0.3g Actinide Resin and 100 mL water sample. LSC counting for 4 hours resulted in a detection limit of 0.03Bq/L (0.8 pCi/L.)
The results obtained by Kwakman’s laboratory were in excellent agreement with the OPRI target value and had significantly less uncertainty than the typical laboratories’ results. His results, shown in Figure 4, were presented at Eichrom’s European Users’ Meeting in Paris, May, 2002.
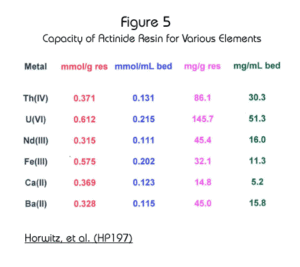
The maximum loading capacity of Actinide Resin for various elements is shown in Figure 5. The resin exhibits a high capacity for actinide elements, in particular hexavalent uranium (50 mg/mL of resin) and tetravalent thorium (30 mg/mL of resin.)
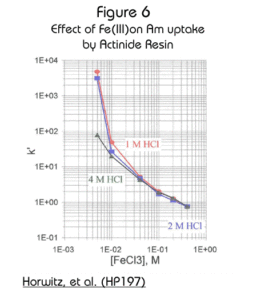
The effect of iron on the retention of the actinides is often a concern in analytical separations. The effect of Fe(III) and Fe(II) on the retention of Am(III) in a variety of HCl solutions is shown in Figures 6 and 7. While the effect of Fe(III) is significant, the effect of Fe(II) is minimal. In samples with suspected high concentrations of iron, the addition of a reducing agent, such as ascorbic acid, will minimize the effect of iron on the retention of americium.
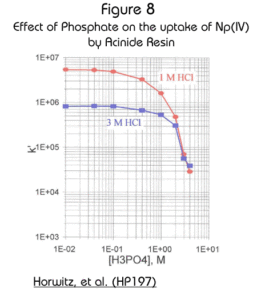 Complexing anions (e.g., sulfate and phosphate) can interfere with the uptake of tetravalent actinides on the Actinide Resin (Figure 8). actinides. Even though the effect is significant, because the magnitude of the k’ is so high to begin with, concentrations of these anions up to 1M result in k’ for Np(IV) of greater than 105 (in either 1M or 3M HCl) and should cause no observable problems in typical analytical schemes. Because the retention of actinides on Actinide Resin is so high, it is often not practical to strip the actinides from the resin with aqueous solutions. Instead it is necessary to dissolve the DIPEX® extractant with isopropanol. See Burnett, W.C., et al. (BW197) and Qu, H., et al. (QH198) for details.
Complexing anions (e.g., sulfate and phosphate) can interfere with the uptake of tetravalent actinides on the Actinide Resin (Figure 8). actinides. Even though the effect is significant, because the magnitude of the k’ is so high to begin with, concentrations of these anions up to 1M result in k’ for Np(IV) of greater than 105 (in either 1M or 3M HCl) and should cause no observable problems in typical analytical schemes. Because the retention of actinides on Actinide Resin is so high, it is often not practical to strip the actinides from the resin with aqueous solutions. Instead it is necessary to dissolve the DIPEX® extractant with isopropanol. See Burnett, W.C., et al. (BW197) and Qu, H., et al. (QH198) for details.
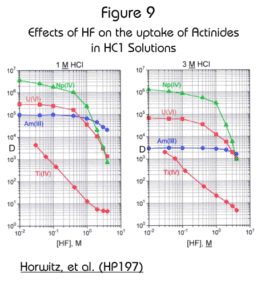 The effect of fluoride (added as HF) is shown in Figure 9. The uptake of actinide elements is not significantly affected by HF. This feature allows for the Actinide Resin to be used as a preconcentrator for actinides directly from soil samples digested in HCl/HF solutions. HF used to dissolve silica in soil samples will not significantly interfere with actinide retention on the Actinide Resin if it’s concentration is held below 1M.
The effect of fluoride (added as HF) is shown in Figure 9. The uptake of actinide elements is not significantly affected by HF. This feature allows for the Actinide Resin to be used as a preconcentrator for actinides directly from soil samples digested in HCl/HF solutions. HF used to dissolve silica in soil samples will not significantly interfere with actinide retention on the Actinide Resin if it’s concentration is held below 1M.
Actinide Resin is available as 50-100 mm or 100-150 µm resin and is sold in bottles or ready to use in prepackaged 2 mL columns (for gravity flow) and 2 mL cartridges for use with Eichrom’s vacuum box system. Click here for part numbers and descriptions.
SOURCE for all published data:
Horwitz, E.P., Chiarizia, R. and Dietz, M.L.,
DIPEX: A New Extraction Chromatographic Material for the Separation and Preconcentration of Actinides from Aqueous Solution, Reactive & Functional Polymers, Vol. 33, pp. 25-36(1997) Actinides in Aqueous using Actinide Resin (HP197)