 Eichrom’s Nickel Resin is based on the traditional dimethylglyoxime (DMG) precipitation chemistry for nickel analysis. The Nickel Resin contains the DMG inside the pores of a polymethacrylate resin. The nickel-DMG precipitate occurs on the resin , where it is held and readily separated from aqueous samples. Figure 1 shows the chemical structure of DMG and the nickel-DMG complex. Applying the DMG chemistry in a column or cartridge format simplifies the separation procedure and allow simultaneous preparation of multiple samples.
Eichrom’s Nickel Resin is based on the traditional dimethylglyoxime (DMG) precipitation chemistry for nickel analysis. The Nickel Resin contains the DMG inside the pores of a polymethacrylate resin. The nickel-DMG precipitate occurs on the resin , where it is held and readily separated from aqueous samples. Figure 1 shows the chemical structure of DMG and the nickel-DMG complex. Applying the DMG chemistry in a column or cartridge format simplifies the separation procedure and allow simultaneous preparation of multiple samples.
Nickel Resin is an 11% (w/w) loading of DMG, with a resin density of 0.25g/mL. The working capacity of the resin for Nickel is 1 mg Ni per mL resin; and is 2 mg per 2 mL pre-packed column or cartridge.
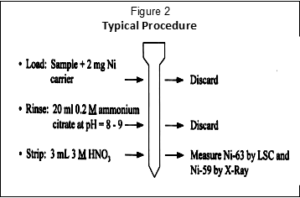 Figure 2 outlines the nickel procedure. The sample, with a 2 mg Ni carrier, is loading from pH 8-9 ammonium citrate solution onto a 2 mL column or cartridge of Nickel Resin. Addition of a 2 mL cartridge of Prefilter Resin below the Nickel Resin can improve the recovery of Ni, particularly from larger sample volumes. After a 20 mL rinse with the same solution, the DMG complex is dissolved and eluted from the column using a small amount of 1.5 – 3M nitric acid. Ni-63 can then be measure by LSC and Ni-59 by gamma spectrometry.
Figure 2 outlines the nickel procedure. The sample, with a 2 mg Ni carrier, is loading from pH 8-9 ammonium citrate solution onto a 2 mL column or cartridge of Nickel Resin. Addition of a 2 mL cartridge of Prefilter Resin below the Nickel Resin can improve the recovery of Ni, particularly from larger sample volumes. After a 20 mL rinse with the same solution, the DMG complex is dissolved and eluted from the column using a small amount of 1.5 – 3M nitric acid. Ni-63 can then be measure by LSC and Ni-59 by gamma spectrometry.
If Fe-55 needs to be measured from the sample then it should be separated prior to loading on the Ni Resin. Refer to Eichrom method FEW01, Fe-55 in Water, where the separation of iron from nickel is made on the TRU Resin.
Table 1
Comparison of Precipitation and Column Methods (Results in µCi/unit)
| Sample Type | Sample Method | Nickel Column | Ratio |
| TL/HS Tank | 2.30E-6 | 2.38E-6 | 0.97 |
| Lab Waste Tank | 2.66E-6 | 2.49E-6 | 1.08 |
| WECT Tank | 4.31E-6 | 4.17E-6 | 1.03 |
| Ni-63 spike | 5.07E-4 | 5.35E-4 | 0.95 |
| Ni-59 spike | 1.00E-2 | 1.07E-2 | 0.93 |
| DAW Smears | 4.18E-2 | 4.63E-2 | 0.90 |
| Radwaste filter | 7.40E-1 | 7.79E-1 | 0.95 |
| RWCU Resin | 1.83E+0 | 2.05E+0 | 0.89 |
D. Cahill, Carolina Power & Light, New Hill NC
Carolina Power & Light evaluated the Nickel Resin. Table 1 presents the results of a side-by-side comparison of the nickel column with the standard DMG precipitation method on a variety of sample types with a range of Ni-63 activity from 10-6 to 100 mCi/unit. Across the whole activity concentration range, the correlation between the precipitation method and the Nickel Resin column was excellent (+/- 10%.)
A decontamination study was also conducted at Carolina P&L using a rad-waste cleanup resin (RWCU Resin) sample. Decontamination factors (the ratio of concentration before and after processing the sample through the Nickel Resin procedure) for various activation and fission products is shown in Table 2.
Table 2
Decontamination Factors (RWCU Resin Sample)
| Sample Type | |
| Cr-51 | >37,000 |
| Mn-54 | 270,000 |
| Co-58 | 110,000 |
| Co-60 | 113,000 |
| Nb-95 | 13,700 |
| Cs-134 | >9,000 |
| Cs-137 | 58,000 |
D. Cahill, Carolina P&L
Table 3 outlines general observations made at Carolina P&L about the column method. An overall comparison of these factors, shows that the column method is significantly faster and easier than the precipitation method. Chemical recoveries of the column method are higher and turnaround time is ~1/3 of the precipitation method. In the case of this study, the column method allowed for the use of an « environmentally friendly » scintillation cocktail that eliminated the generation of mixed waste in the laboratory.
The Nickel Column is manufactured on a 100-150µm resin and is available in bulk and in pre-packaged 2mL columns (for gravity flow separations) and 2 mL cartridges (for use with Eichrom’s vacuum box system). Columns are guaranteed for 6 months from date of purchase. Click here for part number and descriptions.
Table 3
General Observations
| Parameter | Standard Method | Column Method |
| Chemical Yield | 63 +/- 9% | 90 +/- 14% |
| LLD (50mL/30 min) | 1.27E-7 | 6.5E-8 |
| Analysis Time | ||
| Overall | 12 hours | 4 hours |
| Hands-on | 8 hours | 2 hours |
| LSC cocktail | Mixed Waste |
D. Cahill, Carolina P&L, New Hill NC