Actinium-225 Separation
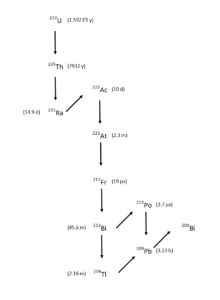
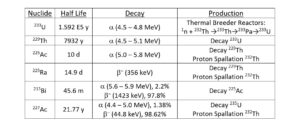 225Ac is a promising therapeutic nuclear medicine radionuclide. Applications include direct treatment with 225Ac conjugated to biolocalization agents and the use of 225Ac as a source of 213Bi. There are several processes available for the production of 225Ac, including decay of 229Th obtained from aged 233U breeder reactor fuel (Figure 1) and proton spallation on 232Th targets. [1-11]
225Ac is a promising therapeutic nuclear medicine radionuclide. Applications include direct treatment with 225Ac conjugated to biolocalization agents and the use of 225Ac as a source of 213Bi. There are several processes available for the production of 225Ac, including decay of 229Th obtained from aged 233U breeder reactor fuel (Figure 1) and proton spallation on 232Th targets. [1-11]
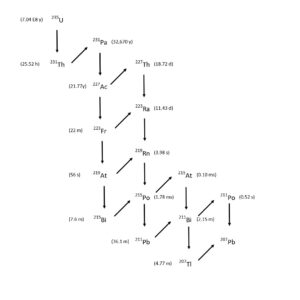
Both of these methods require separation of 225Ac and 225Ra from relatively large amounts of Th (5-50 grams). 225Ra, the immediate parent of 225Ac can be used as a source for additional 225Ac after a short period of ingrowth. The production of 225Ac from 225Ra is particularly important for the 232Th spallation route, as a source of 225Ac without the long lived 227Ac impurity contained in the 225Ac produced directly from spallation (Figure 2). [1, 4 6-11]
There are two main routes for separation of the large mass of Th from the Ac and Ra: 1) dissolution in HNO3 -HF and selective extraction of Th (rejecting Ac/Ra) [1,2,4,6,11] and 2) complexation of Th with sulfate or citrate to form anionic complexes and selective extraction of Ac and Ra by cation exchange resin. [7-10]
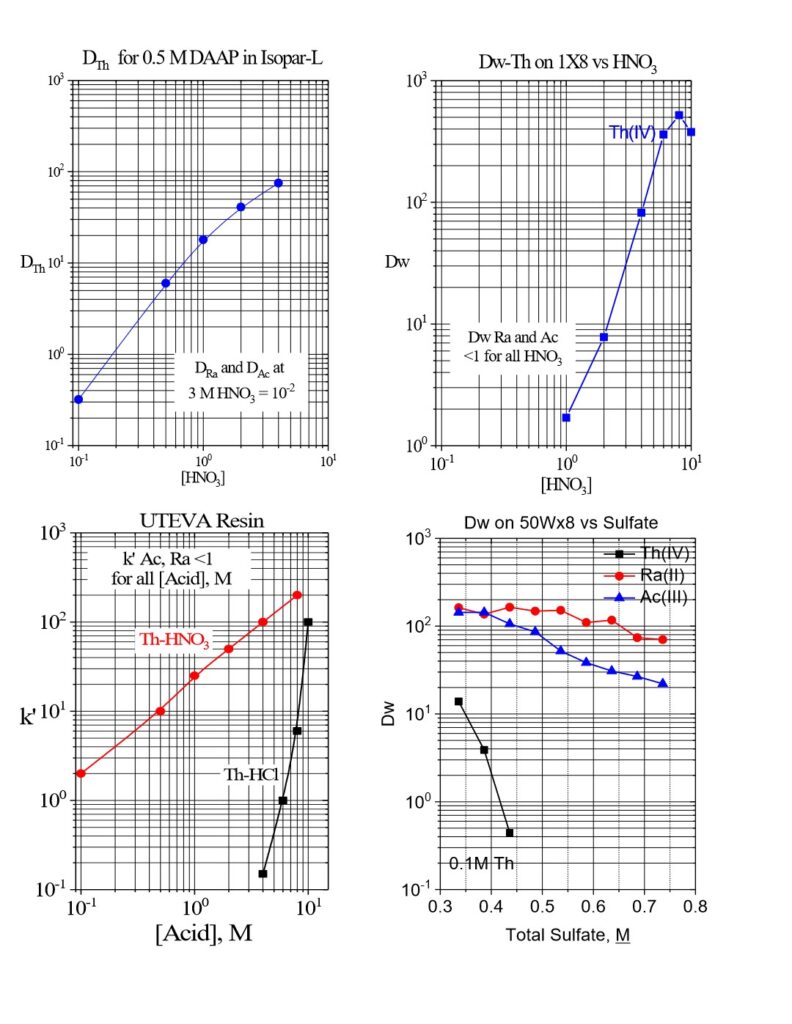
In route 1, Th is dissolved in HNO3 with a trace amount of HF to speed and complete dissolution of thorium metal. Th is then extracted by solvent extraction with organophosphorus extractants, such as diamyl(amyl)phosphonate (DAAP), or chromatography using gel type or macroporous anion exchange resins (Figure 3). The gel type resins offer higher capacity than the macroporous materials (1.7-2.0 vs 1.0-1.5 meq/mL), while the macroporous resins exhibit higher physical stability and resistance to shrinking and swelling while cycling from the high HNO3 (6-8 M) during loading of Th and low HNO3 (0.01 – 0.05 M) during recovery of Th.
Because a large mass of Th is being extracted (5-50 grams), several solvent extraction stages (3 stages of 1-2 L of 0.5M DAAP) or relatively large columns of anion exchange resin (1-2 L) are required for near complete extraction of Th. High concentrations of HNO3 (~8M) are required for efficient retention of Th by anion exchange, and large volumes of eluate (1-2 L) are required to pass the Ac and Ra through the large anion exchange columns. Therefore, evaporation of the Ac/Ra fraction may be required to reduce the HNO3 concentration and volume prior to subsequent 225Ac/Ra purification steps.
In route 2, preparation of the thorium source material for selective extraction of Ac and Ra may also begin with the dissolution of thorium metal with HNO3-HF or HCl-HF, evaporation and redissolution in ammonium citrate or H2SO4 or by direct dissolution by H2SO4-HF. [8.9,12-15] From sulfate or citrate, under the correct conditions, Ac and Ra can be selectively extracted onto 50Wx8 gel-type cation exchange resin, while anionic Th species are rejected (Figure 3).

Figure 4. Dissolution of (a) Th metal powder to form (b) warm thorium sulfate white powder (c) clear thorium sulfate solution upon cooling to room temperature.
Dissolution of Th metal by H2SO4-HF is slower than dissolution in HNO3-HF, but avoids evaporation steps. The Th metal is first converted to a fluffy white Th(SO4)x powder with warm H2SO4 with a trace of HF (Figure 4). Upon cooling and diluting the solution with water, the Th(SO4)x dissolves.
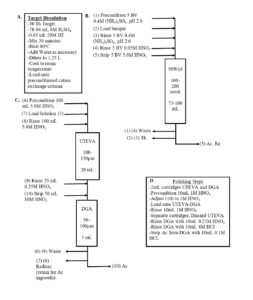
The selective extraction of Ac and Ra while rejecting the large mass of Th, allows the use of relatively small columns of cation exchange resin (50-100 mL). After rinsing to provide additional decontamination from Th, the Ac and Ra are recovered with 150 – 300 mL of 5-6M HNO3, which can be directly processed by the subsequent separation processes without evaporation or feed adjustment (Figure 5).
Following removal of the bulk Th, the Ac/Ra fraction is further purified by ion exchange and extraction chromatography to remove any remaining traces of Th or spallation or decay byproducts (Figure 5C-5D). Further purification methods include anion exchange and UTEVA resin for additional Th removal and removal of spallation byproducts (Pa, U, Po) and DGA normal resin for the separation of Ac and Ra and removal of spallation byproducts (La, Ce, Po, Ba, Sr, etc…) and reagent impurities (Al, Fe, Ca, etc…). [8,9,16]
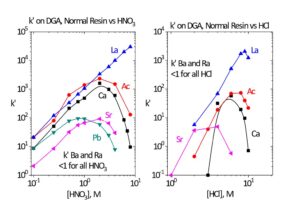
Ultimately, in many 225Ac production methods, 225Ac is purified and concentrated using DGA resin and recovered in dilute HCl. One important impurity that must be considered for separations on DGA resin is calcium. When loading DGA from 1-3M HNO3 and stripping with dilute HCl, Ca will co-elute with Ac (Figure 6). Therefore, rinses of the DGA resin with 9M HCl or 8M HNO3 are needed to remove Ca impurities (Figure 7). [9]
In the spallation production route, rare earth impurities, such as 140La(140Ba), 139Ce, 141Ce, and 144Ce, are produced. These rare earth impurities must be separated from 225Ac fraction. One effective method for the group separation of rare earth metal ions from Ac is elution from DGA, normal with 9-10 M HNO3 (Figure 6). [7] At the high HNO3 concentration, the retention of Ac decreases, while the retention of rare earth metal ions remains high. The Ac fraction, in 9-10 M HNO3, can then be diluted to 6-8M HNO3 and processed through another DGA, normal cartridge to concentrate the Ac and convert to dilute HCl (Figure 5).
References
1] Aliev, R.A., Ermolaev, S.V., Vasiliev, A.N., Ostapenko, V.S., Lapshina, E.V., Zhuikov, B.L., Zakharov, N.V., Pozdeev, V.V., Kokhanyuk, V.M., Myasoedov, B.F., Kamykov, S.N. 2014. Isolation of Medicine-Applicable Actinium-225 from Thorium Targets Irradiated by Medium Energy Protons. Solv. Extr. Ion. Exch. 32(5), 468-477.
2] Boll, R.A., Malkemus, D., Mirzadeh, S. 2005. Production of Ac-225 for alpha particle therapy. Appl. Radiat. Isot. 62, 667-679.
3] Bond, A.H., Horwitz, E.P., McAlister, D.R., 2006. A Multicolumn Selectivity Inversion Generator for the Production of High Purity Actinium-225 for Use in Therapeutic Nuclear Medicine, United States patent number 7,087,206. (Licensed to Northstar Medical Radioisotopes)
4] Ermolaev, S.V., Zhuikov, B.L., Kokhanyuk, V.M., Matushko, V.L., Kalmykov, S.N., Aliev, R.A., Tananaev, I.G., Myasoedov, B.F. 2012. Production of actinium, radium, and thorium isotopes from natural thorium irradiated with protons up to 141 MeV. Radiochim. Acta. 100, 223-229.
5] Harvey, J.H., Nolen, J.A., Kroc, T., Gomes, I., Horwitz, E.P., McAlister, D.R. 2009. Alternative Methods for the Production of Ac-225. 10th International Symposium of the International Isotope Society, Chicago, IL, June 14-18.
6] Harvey, J.H., Nolen, J., Vandergrift, G., Kroc, T., Gomes, I., McAlister D.R., Horwitz, E.P. 2011. Production of Actinium-225 via High Energy Proton Induced Spallation on Thorium-232. Final Technical Report DE-SC0003602. https://www.osti.gov/scitech/servlets/purl/1032445/
7] Mastren, T., Radchenko, V., Owens, A., Copping, R., Boll, R., Griswold, J.R., Mirzadeh, S., Wyant, L.E., Brugh, M., Engle, J.W., Nortier, F.M., Birnbaum, E.R, John, K.D., Fassbender, M.E. 2017. Simultaneous Separation of Actinium and Radium Isotopes from a Proton Irradiated Thorium Matrix. Nature Scientific Reports, 7, 8216. doi:10.1038/s41598-017-08506-9
8] McAlister, D.R., Horwitz, E.P. 2018. Selective Separation of Radium and Actinium from Bulk Thorium Target Material on Strong Acid Cation Exchange Resin from Sulfate Media, Applied Radiation and Isotopes, 140, 18-23.
9] McAlister, D.R., Horwitz, E.P., Perron, R., Gendron, D., Causey, P., Harvey, J.T., “Selective Separation of Radium and Actinium from Bulk Thorium Target Material,” 11th International Symposium on Targeted Alpha Therapy, Ottawa, Ontario, Canada, April 1-4, 2019.
10] Radchenko, V., Engle, J.W., Wilson, J.J., Massen, J.R., Nortier, F.M., Taylor, W.A., Birnbaum, E.R., Hudston, L.A., John, K.D., Fassbender, M.E., 2015. Application of ion exchange and extraction chromatography to the separation of actinium from proton-irradiated thorium metal for analytical purposes, J. Chromatogr. A. 1380, 55-63.
11] Zhuikov, B.L., Kalmykov, S.N., Ermolaev, S.V., Aliev, R.A., Kokhanyuk, V.M., Matushko, V.L., Tananaev, I.G., Myasoedov, B.F. 2011. Production of 225Ac and 225Ra by Irradiation of Th with Accelerated Protons. Radiochemistry, 53(1), 73-80.
12] Allen, K.A., McDowell, W.J. 1963. The Thorium Sulphate Complexes from Di-n-decylamine Sulphate Extraction Equilibria. J. Phys. Chem. 67, 1138-1140.
13] Keskar, M., Kasar, U.M., Singh Mudher, K.D., 2000. Solid State Reactions of UO2, ThO2 and their Mixed Oxides with Sulphates of Potassium, J. Nucl. Mater. 282, 146-151.
14] Zebroski, E.L., Alter, H.W., Heumann, F.K. 1951 Thorium Complexes with Chloride, Fluoride, Nitrate, Phosphate and Sulfate. J. Am. Chem. Soc. 73(12), 5646-5650.
15] Ziehlen, A.J. 1959. Thermodynamics of the Sulfate Complexes of Thorium. J. Am. Chem. Soc., 81(19), 5022-5028.
16] Horwitz, E.P., McAlister, D.R., Bond, A.H., Barrans, Jr., R.E. 2005. Novel Extraction Chromatographic Resins Based on Tetraalkyldiglycolamides: Characterization and Potential Applications. Solv. Extr. Ion Exch., 23, 319-344.