Lutetium-177 Separation
The beta emitting isotope 177Lu has garnered interest in nuclear medicine for targeted radionuclide therapy.[1,2] 177Lu is produced by neutron irradiation of Yb targets.
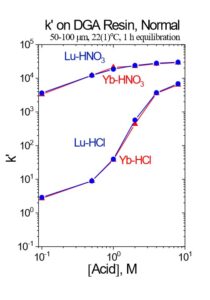
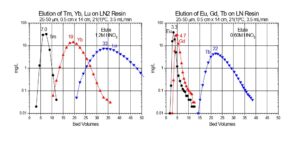
Due to the small separation factors between adjacent rare earth elements, the separation of 177Lu from 10-300 mg of Yb target material requires relatively long columns, small particle size resin, elevated temperature and multiple separation columns to achieve the desired high specific activity final products.[1,3,4] Two main chromatographic systems have been applied to the separation of adjacent rare earths: 1) Extraction chromatographic resins (EXC) based on organophoshorus extractants (HDEHP = LN Resin and HEH[EHP] = LN2 Resin) from mineral acids (HNO3 and HCl) and 2) Strong acid cation exchange resin (50Wx8 or MP50) from alpha hydroxybutyric acid (α-HIBA).[1]
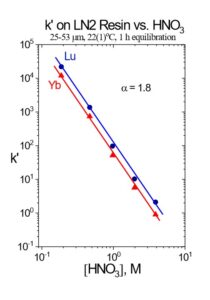
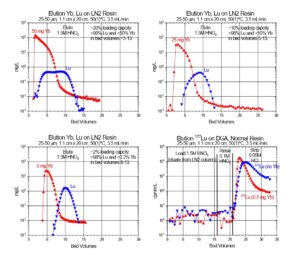
In the EXC separation schemes, LN2 or LN resin is used to perform the bulk of the Lu/Yb. The separation factors for adjacent rare earth elements is normally small, ranging from 1.5 to 4.9.[5] The separation factor is 1.8 for Lu/Yb on LN2 (Figure 1). Separation factors for LN and LN2 resins are normally very similar, and heavier (higher atomic number) rare earth metal ions are retained more strongly than lighter (lower atomic number) rare earth metal ions. The less acidic HEH[EHP] extractant on LN2 allows recovery of rare earths in more dilute acid than the more acidic HDEHP on LN resin. Therefore, LN2 resin is often used for separation of heavier rare earth elements (Dy-Lu), Y and Sc, while LN is more often used for lighter rare earth elements (La-Tb), and Ac. When the loading of LN or LN2 resin is low (<5% functional capacity) adjacent rare earth elements can be separated efficiently using a single column of f-grade (25-50 mm) resin (Figure 2).[3,5]
With relatively large targets 50-300 mg of Yb or Gd, it may be impractical to pack a single column large enough to keep the functional capacity below 5%. Furthermore, a single large column may not provide adequate decontamination of the desired radionuclide from the target material to achieve the high specific activities and “no carrier added” (n.c.a) designation required for efficient labeling of 177Lu to biolocalization agents for targeted radionuclide therapy. In these cases, a series of 2-3 LN2 resin columns, operated at higher capacity, can provide a more efficient separation (Figure 3).[1,3]
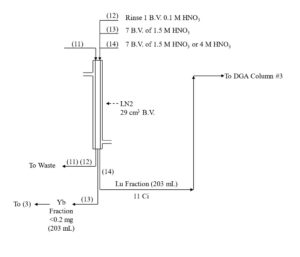
Yb and Lu are loaded onto the first LN2 column from 0.05-0.10 M HNO3 consuming ~20% of the column’s functional capacity and eluted with 1.5M HNO3. Yb is less strongly retained an elutes first, while >99% Lu and <50% of Yb is recovered in bed volumes ~3-13. Bed volumes 1-3, containing primarily Yb may be collected to preserve the Yb target material for recycling. High valence impurities, such as Th(IV), Zr(IV), Hf(IV) and U(VI) are retained strongly and remain on the LN2 resin. Fe(III) may co-elute with Lu and must be separated in subsequent steps.
DGA resin, normal is used between successive LN2 columns to concentrate the Lu and convert from the relatively high concentration of HNO3 used to elute the LN2 and LN and the low concentration of HCl required to load the subsequent LN2 column (Figure 3). Utilizing the DGA resin, normal eliminates the need for evaporations, enables the rapid separation of 177Lu using up to 3 LN2 or LN column separations, and helps to remove reagent impurities, such as Al, Ca, and Fe.[3,6]
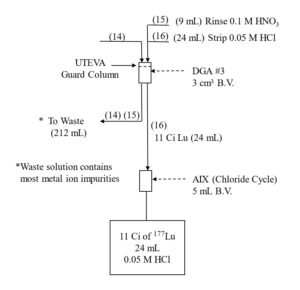
The Lu fraction from the first LN2 column is loaded directly onto a column of DGA resin, normal, rinsed with 0.10 M HNO3 to reduced the acidity, and then Lu is recovered using 0.05M HCl (Figure 4). The Lu in 0.05M HCl is then loaded onto the second LN2 resin column which reduces the Yb content to 98% of the Lu. Following concentration on DGA, resin, normal, a third LN2 resin column completes the Lu/Yb separation, and the 177Lu is concentrated on a final DGA resin, normal cartridge.
As long as there is a significant mass of Yb (>0.2 mg) remaining with the 177Lu fraction, recovery of 177Lu from the DGA, resin, normal with dilute HCl is very efficient. However, after the final LN2 separation, when nearly all of the Yb has been removed, the recovery of 177Lu from the DGA resin can be retarded by the small amount of extractant that has leached from the LN2 resin.[3] The acidic HEH[EHP] extractant leached from LN2 is adsorbed onto the DGA resin and complexes 177Lu, leading to a broad elution band and lower recovery from dilute HCl.
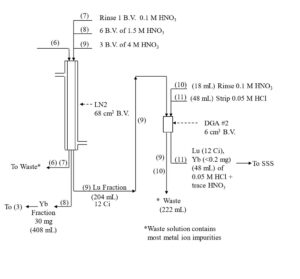
The impact of the LN2 extractant leach is minimized by introducing a small guard cartridge of UTEVA resin above the final DGA Resin column. The UTEVA resin adsorbs the HEH[EHP] and is removed prior to stripping the 177Lu from DGA, leading to more complete recovery of 177Lu in a small volume of dilute HCl. Passing the final 177Lu eluate from the DGA resin column through a small column of anion exchange resin in the chloride form (A8-F-CL, strong base anion exchange) helps to remove any residual nitrate. A flowsheet for the 177Lu separation method is provided in Figures 5-8.
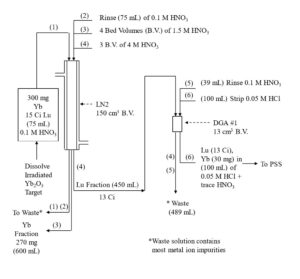
Figure 5. Initial separation of Lu-177 from Gd target material on LN2 resin with concentration on DGA, normal resin.
References
[1] Dash, A., Raghavan, M., Pillai, A. Knapp, F.F. 2015. Production of 177Lu for Radionuclide Therapy: Available Options, Nucl. Med. Mol. Imaging, 49, 85-107.
[2] Lehenberger, S., Barkhausen, C., Cohrs, S., Fischer, S., Grunberg, J., Hohn, A., Koster, U., Schibli, R., Turler, A., Zhernosekov, K., 2011. The low energy b- and electron emitter 161Tb as an alternative to 177Lu for targeted radionuclide therapy, Nucl. Med. Bio., 38, 917-924.
[3] Horwitz, E.P. McAlister, D.R. Bond, A.H. Barrans, Jr., R.E. Williamson, J.M. 2005. A Process for the Separation of 177Lu from Neutron Irradiated 176Yb Targets, Applied Radiation and Isotopes, 63, 23-36.
[4] Mirzadeh, S., Du, M., Beets, A. L., Knapp, Jr., F. F., April 6, 2004. Method for preparing high specific activity 177Lu. United States Patent No. 6,716,353.
[5] McAlister, D.R. Horwitz, E.P. 2007. The Characterization and Novel Applications of Extraction Chromatographic Materials Containing Bis(2-ethyl-1-hexyl)phosphoric Acid, 2-ethyl-1-hexylphosphonic acid, mono 2-ethyl-1-hexyl ester and 2,4,4-trimethyl-1-pentylphosphinic acid, Solv. Extr. Ion Exch., 25(6), 757-769.
[6] Horwitz, E.P. McAlister, D.R. Bond, A.H. Barrans, Jr., R.E. 2005. Novel Extraction Chromatographic Resins Based on Tetraalkyldiglycolamides: Characterization and Potential Applications,” Solv. Extr. Ion Exch., 23, 319-344 (2005).