 One of the more versatile of Eichrom’s analytical products is TEVA Resin. It has been applied on a routine basis to the analysis of technetium, the measurement of the tetravalent actinides, and the separation of americium from lanthanides. It is used alone or can be readily combined with other resins to perform more elaborate separations of multiple analytes.
One of the more versatile of Eichrom’s analytical products is TEVA Resin. It has been applied on a routine basis to the analysis of technetium, the measurement of the tetravalent actinides, and the separation of americium from lanthanides. It is used alone or can be readily combined with other resins to perform more elaborate separations of multiple analytes.
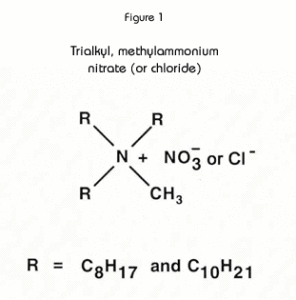
The active component of the TEVA Resin is an aliphatic quaternary amine. (See Figure 1.) with properties similar to those of typical strong base anion exchange resins. However, because the functional groups are in a liquid form, rather than fixed to a polymer backbone (as with IX resins), the liquid anion exchanger has greater flexibility to coordinate around target anions. Consequently, the uptake of anions is generally higher on TEVA Resin, and lower acid concentrations may often be empolyed to achieve separations.
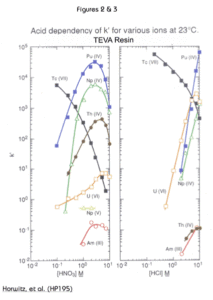 The uptake of selected metal ions on TEVA Resin from HNO3 and HCl is presented in Figures 2 and 3. Tetravalent plutonium, neptunium and thorium show maximum uptake in the region of 2 M to 4 M nitric acid. In this acid concentration range, hexavalent uranium and trivalent americium are not well retained. Therfore, TEVA Resin can readily separate the tetravalents from the other actinides. Due to this selectivity, TEVA is often utilized as the first resin in methods employing multiple stacked cartridges or columns in tandem separations.
The uptake of selected metal ions on TEVA Resin from HNO3 and HCl is presented in Figures 2 and 3. Tetravalent plutonium, neptunium and thorium show maximum uptake in the region of 2 M to 4 M nitric acid. In this acid concentration range, hexavalent uranium and trivalent americium are not well retained. Therfore, TEVA Resin can readily separate the tetravalents from the other actinides. Due to this selectivity, TEVA is often utilized as the first resin in methods employing multiple stacked cartridges or columns in tandem separations.
The decrease in k’ for the tetravalent actinides for HNO3 concentrations greater than 2-4M is due to competition from nitrate anions for complexation sites on the resin.
The differences between the uptake curves for nitric and hydrochloric acid can be exploited to separate certain actinides from each other. For example, all the tetravalent actinides can be loaded from 3M nitric acid. Then, by switching to 6-9M HCl, Th(IV) can be selectively eluted from TEVA, while Pu(IV) and Np(IV) remain on the column. Pu and Np can then be recovered together or separately through the use of the appropriate reducing agent, complexants and acid concentrations.
The retention of Tc(VII), pertechnetate, is also shown in figures 2 and 3. TEVA resin retains pertechnetate strongly in solutions of dilute HNO3 or HCl, and Tc can then be recovered from TEVA using 8-10M HNO3. For applications where the 8-10M HNO3 eluate is not desireable for Tc analysis, Eichrom’s WBEC (weak base EXC resin) may be a useful alternative. Since the WBEC only acts as an anion exchanger when protonated from acid solution, Tc can be recovered from WBEC using alkaline solutions, such as 1M NH4OH.
The use of TEVA Resin in the analysis of Tc has become an industry standard. TEVA Resin is also available in a disc format which can tolerate flow rates of up to 200 mL/min when used for Tc analysis. Following the loading of Tc onto the disc, the disc can be rinsed, dried and added directly to scintillation cocktail.
 Although not shown on Figures 2 and 3, technetium uptake on TEVA is also high from alkaline solutions. Work performed by Darrin Mann’s laboratory at K-25 in Oak Ridge demonstrated that the TEVA Resin could be used to isolate Tc-99 from a variety of matrices including alkaline solutions and neutralized acids. (See reference MA193.)
Although not shown on Figures 2 and 3, technetium uptake on TEVA is also high from alkaline solutions. Work performed by Darrin Mann’s laboratory at K-25 in Oak Ridge demonstrated that the TEVA Resin could be used to isolate Tc-99 from a variety of matrices including alkaline solutions and neutralized acids. (See reference MA193.)
Horwitz, et al. reported the data in Figures 2 and 3 from studies performed with experimental batches of TEVA Resin. Eichrom’s commercial product conforms to established specifications that ensure proper performance of Eichrom issued methods. Please refer to our product specificationsfor details.
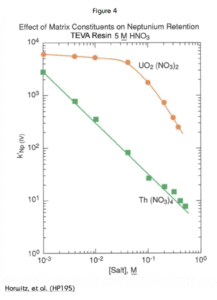
Figure 4 shows the effect of U(VI) and Th(IV) content on the uptake of Np(IV) by TEVA Resin from 5M HNO3. While the effect of Th(IV) is significant and linear, U(VI) doesn’t have much negative impact on Np retention until the concentration reaches 0.05M. In a 15 mL load solution, for example, this would correspond to about 180 mg of U(VI).
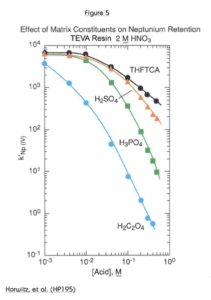 The matrix effects of various polyatomic anions on Np(IV) uptake by TEVA Resin from 2M HNO3 are shown in Figure 5. Oxalate shows the strongest effect on neptunium uptake. Oxalato complexes form readily with the tetravalent actinides, and these complexes are not extracted by the TEVA Resin. Oxalates are often used in stripping solutions of tetravalent actinides from TEVA Resin.
The matrix effects of various polyatomic anions on Np(IV) uptake by TEVA Resin from 2M HNO3 are shown in Figure 5. Oxalate shows the strongest effect on neptunium uptake. Oxalato complexes form readily with the tetravalent actinides, and these complexes are not extracted by the TEVA Resin. Oxalates are often used in stripping solutions of tetravalent actinides from TEVA Resin.
Phosphate is commonly found in a variety of sample matrices. The effect of phosphate on all tetravalent actinides (Pu, Th, and Np) would be similar, but since Th is the least strongly retained by TEVA Resin, it is most readily affected by high levels of phosphate in a sample. The addition of aluminum to the load solution will reduce this effect. Phosphate will preferentially complex aluminum, leaving the tetravalent actinides free to form extractable nitrato complexes.
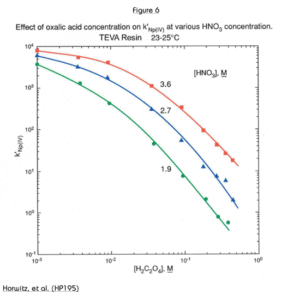 Another solution to the problem of anionic matrix interferences is shown in Figure 6. In the case of oxalate, increasing the nitric acid concentration of the load solution from 2M to 3.5M increased the uptake of Np(IV) by more than an order of magnitude.
Another solution to the problem of anionic matrix interferences is shown in Figure 6. In the case of oxalate, increasing the nitric acid concentration of the load solution from 2M to 3.5M increased the uptake of Np(IV) by more than an order of magnitude.
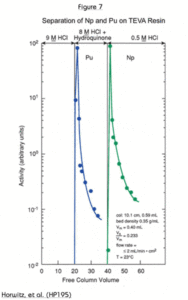 Figure 7 shows the separation of Pu from Np using TEVA Resin. This is accomplished by the selective reduction of plutonium from tetravalent to Pu(III). In this valence state, Pu behaves like Am, which is not retained on TEVA from any concentration of nitric or hydrochloric acid. In the example in Figure 7, plutonium is reduced with hydroquinone, although other reagents such as ammonium iodide and ferrous sulfamate may also be used.
Figure 7 shows the separation of Pu from Np using TEVA Resin. This is accomplished by the selective reduction of plutonium from tetravalent to Pu(III). In this valence state, Pu behaves like Am, which is not retained on TEVA from any concentration of nitric or hydrochloric acid. In the example in Figure 7, plutonium is reduced with hydroquinone, although other reagents such as ammonium iodide and ferrous sulfamate may also be used.
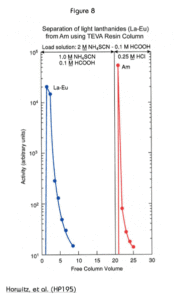
Another interesting use of the TEVA Resin is to separate americium from the rare earth elements. Figure 8 shows that the rare earths may be eluted as a group in a load solution of 1.0 M ammonium thiocyanate and 0.1 M formic acid. Americium is retained under these conditions and can be eluted later with hydrochloric acid. Although Figure 8 shows the elution of Am with 0.25M HCl, it has been later shown that 2M HCl provides a more effective and reproducible recovery of Am from TEVA resin following loading from thiocyanate. A complete step by step method for the actinide/rare earth separation is provided in Eichrom methods SPA03 and SPA03VBS.
 TEVA Resin is manufactured in three particle sizes (20-50 µm, 50-100 µm, and 100-150 µm) and is sold in bottles or ready to use in prepackaged columns (for gravity flow), cartridges (for vacuum assisted flow), or in a disc format for Tc-99 analysis of large volume water samples. Click here for part numbers and descriptions.
TEVA Resin is manufactured in three particle sizes (20-50 µm, 50-100 µm, and 100-150 µm) and is sold in bottles or ready to use in prepackaged columns (for gravity flow), cartridges (for vacuum assisted flow), or in a disc format for Tc-99 analysis of large volume water samples. Click here for part numbers and descriptions.
Source: Horwitz, E.P., Dietz, M.L., Chiarizia, R., Diamond, H., Maxwell III, S.L., and Nelson, M., « Separation and preconcentration of actinides by extraction chromatography using a supported liquid anion exchanger: Application to the characterization of high-level nuclear waste solutions, » Analytica Chimica Acta, 310 (1995) 63-78. (HP195)