Iron Control Process for Copper Electrolyte
Process Fact Sheet
Process Description
Eichrom Industries developed the Iron Control Process for Copper Electrolyte to provide copper mining facilities with a simple ion exchange system for extracting iron from copper electrolyte in the solvent extraction -electrowinning (SX/EW) process. The system can produce operational benefits resulting in significant operational cost savings in a number of ways, including: Reduced cobalt losses from electrolyte bleeding Improved current efficiency Lowered sulfuric acid losses Increased copper cathode production.
The Process
As copper is depleted from the CuSO4-H2SO4 electrolyte solution during copper electrowinning, the concentration of iron remaining in the solution increases. This build up of iron can result in a loss of current efficiency in the electrowinning process due to the continuous oxidation/reduction of Fe2+ to Fe3+. The conventional method for controlling the iron concentration has been to periodically or continuously bleed a portion of the iron-rich, copper-depleted electrolyte from the circuit and replace it with copper-rich electrolyte solution. In the copper electrowinning process, lead based alloys are used as anodes. Soluble cobalt at a concentration ranging from 50 – 200 ppm is added to the copper electrolyte to control the corrosion of the anode and prevent lead contamination of the copper cathode. During bleed of copper electrolyte to control iron concentration, cobalt is also lost from the system. Cobalt must be continually added to the electrolyte to make up for losses through the bleed stream. Cobalt replacement to control lead anode corrosion is a major operating expense in copper SX/EW plants.
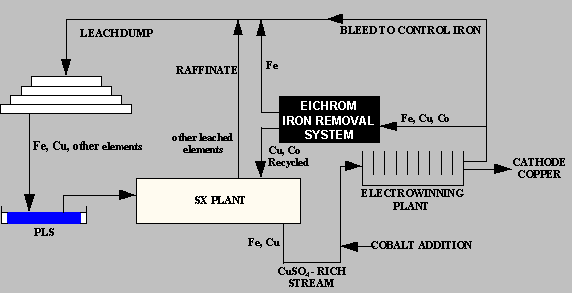
The Resin
The cost effective removal of iron from copper electrolyte is made possible through the use of Eichrom’s Diphonix® resin. This resin resembles a conventional ion exchange resin in its use of sulfonic acid functional groups on a styrene/divinylbenzene matrix. Unlike conventional ion exchange materials, Diphonix resin contains additional complexing ligands that form stable complexes with certain classes of elements, notably Fe(III). These ligands tend not to bind mono- or divalent cation species present in highly acidic streams, establishing a means to selectively remove the iron. Systems using Diphonix resin look and operate identically to systems using conventional ion exchange resins, with the advantage of enhanced performance under challenging operating conditions. Under the ~150 g/L sulfuric acid solution conditions, retention of trivalent (and higher valence) cations is preferred relative to mono-valent and di-valent species. The resin’s selectivity for ferric iron over divalent copper and cobalt ions, combined with operating characteristics typical of conventional sulfonic acid resins, suggests the use of Diphonix resin for the control of iron in copper electrolyte.
Process Chemistry & Laboratory Study
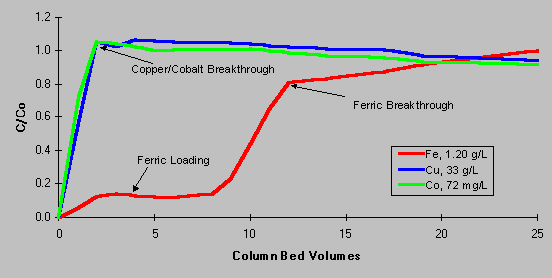
As shown on the loading curve below, Diphonix resin has the ability to retain iron from plant electrolyte solution, but does not complex copper and cobalt. C/Co represents the ratio of processed solution concentrations (column effluent) to initial metal concentrations (column feed). A C/Co value less than one indicates species retention, C/Co equal to one indicates no retention, C/Co greater than one indicates displacement of a previously retained metal into the effluent. As the first bed volume of test solution reaches the outlet sample point, the value for C/Co rapidly approaches one for copper and cobalt, indicating that Diphonix resin does not retain these species. Iron C/Co values remain around 0.15 – 0.20 until the exchange capacity of the resin is exceeded. The reason for the “slippage” of about 20% of the iron through the resin is explained by the fraction of iron present in the ferrous oxidation state. As a divalent species, ferrous iron reacts similarly to cobalt and copper. This behavior of ferrous ions suggests a method whereby the iron exchanged onto the resin could be subsequently removed. A novel, sulfate-based stripping method was developed to remove complexed iron from the resin. This chemistry is completely compatible with leaching and SX operation, allowing process effluent to be easily disposed.
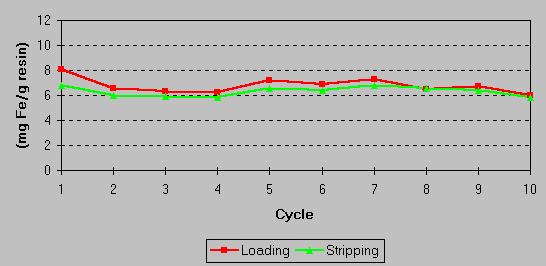
The effectiveness of the iron stripping and the overall capacity of the resin were confirmed in laboratory studies. No loss of resin capacity was observed following a number of load/strip cycle tests. A continuing exchange capacity of 6 – 8 grams of iron per liter of resin results from a stripping efficiency of 70 – 80%. This is shown in the graph below:
Plant Demonstration Studies
Several in-plant demonstrations of this process confirmed the laboratory findings. Key findings included:
Demonstration of Consistent Iron Removal
In all field trials conducted, the loading of resin demonstrated was 8 – 10 grams of iron per liter of resin. The consistent loading achieved during extended plant trials indicates no fouling agents in the electrolyte solution were present to inhibit resin performance.
Process Optimization
The wide range of electrolyte chemistries observed during field work was accommodated by modifying the process to meet specific system needs. This allows for more precise sizing of the production-scale process equipment required to implement the process, resulting in economical system performance.
Successful Use of Continuous Ion Exchange Exchange Equipment
The continuous ion exchange equipment fills the needs of efficiently using Diphonix resin to treat copper electrolyte solution. The equipment has the flexibility to allow any fraction of the resin inventory to be allocated to loading, with the remainder being regenerated.
Getting Started
Based on the experience derived at a number of mine sites, your Eichrom engineering team has the knowledge and experience to quickly formulate a plan for the treatment of your process. In most cases we will recommend conducting a one to two month in-plant trial to define the specific operating conditions of your electrolyte. This allows for optimal sizing of the full-scale system, usually resulting in considerable savings in resin and hardware purchase costs. A starting point for our work is based on “resin productivity”, the mass of iron that can be accumulated by a known volume of resin in a given time period. Based on plant input of daily iron loading values, sizing calculations can be performed to derive the appropriate resin volume for the specific application. Note that resin productivity increases as the rotation time for the continuous ion exchange equipment decreases. This provides operational flexibility for situations where the plant seeks to deplete the existing iron concentration in the electrolyte, then run at a lower iron value and derive improved current efficiency credits. The equipment can be sized for the anticipated daily loading, but initially run at a faster rotation rate. After the existing iron “inventory” is removed, operation can proceed at design conditions. The basic system parameters required to conduct an analysis of the benefits for iron control for your system are:
- Existing or anticipated iron loading from SX process
- Current iron concentration in electrolyte
- Costs of cobalt, electricity, replacement acid
- Existing or planned bleed rate