Lead-201/203/212
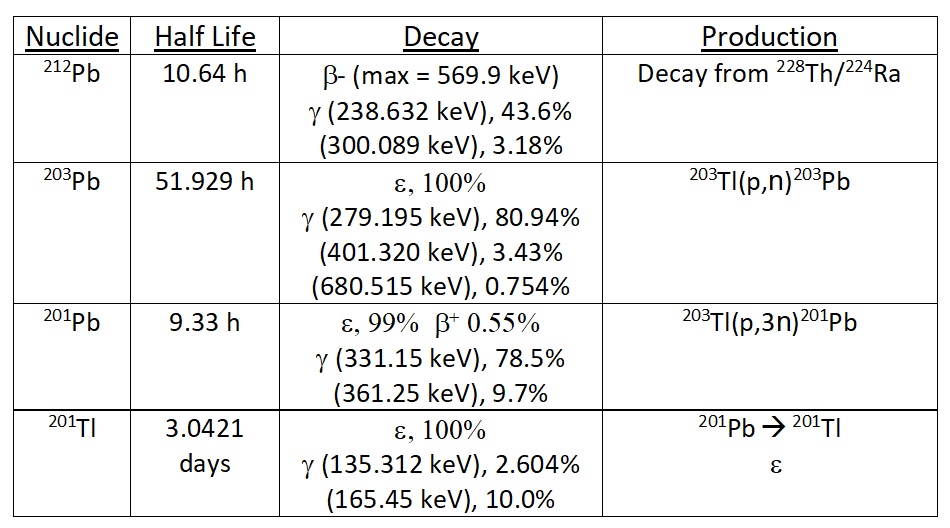
There are several isotopes of lead that are important in nuclear medicine (Table 1). 201Pb, produced by proton irradiation of isotopically enriched thallium targets, is the parent of 201Tl used in heart scans.[1] 203Pb, also produced by proton irradiation of thallium targets, is the diagnostic radioisotope used as a theranostic pair in conjunction with 212Pb therapy in the imaging and treatment of various types of cancer and other disease.[2,3] 212Pb is typically produced by elution of a small volume of 2M HCl through a generator prepared by the loading of 224Ra onto macroporous cation exchange resin.[4]
Following irradiation, Tl targets can be dissolved with HNO3, and Pb resin used to selectively retain 201Pb or 203Pb. [5,6] The thallium target material and most irradiation byproducts and other impurities pass through the Pb resin unretained, and rinsing the column with HNO3 provides additional purification of the Pb from Tl and other impurities. Lead is then recovered from the Pb resin with 6-8M HCl or 1M ammonium acetate.
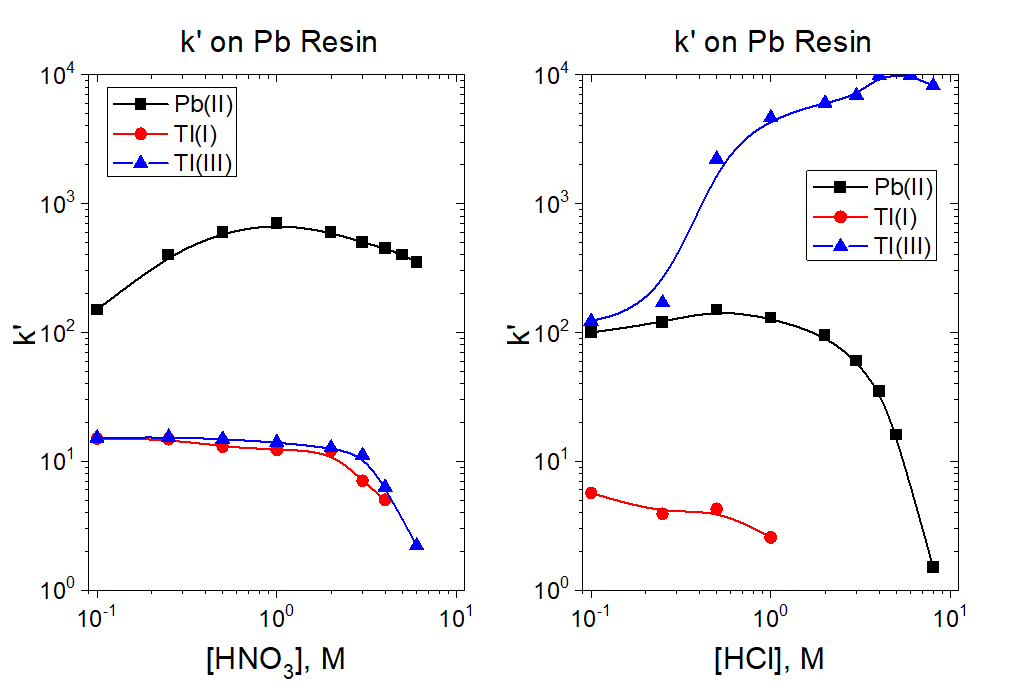
The k’ values for Pb and Tl on Pb Resin from from HNO3 and HCl are presented in Figure 1. Thallium has two oxidation states that are relatively stable in aqueous solution, Tl(I), thallous ion, and Tl(III), thallic ion.[7] The behavior of these oxidation states is very different on Pb Resin, particularly from HCl. Ascorbic acid can be used to reduce thallium to Tl(I), while chlorine water or H2O2-HCl can be used to oxidize thallium to Tl(III). Reduction to Tl(I) helps to ensure good separation of the thallium target material from Pb on Pb Resin.
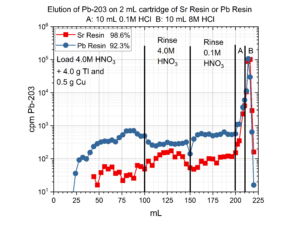
The separation of 203Pb from 4.0 grams of Tl metal dissolved in HNO3 is presented in Figure 2. Using a 2 mL cartridge of Sr Resin or Pb resin, good recovery of 203Pb and nearly complete removal of Tl is achieved. In this example, 203Pb is recovered from the Sr and Pb resins using a small volume of 8M HCl. However, Thallous chloride, TlCl, has a relatively low solubility (Ksp = 1.86E-4, which corresponds to ~4mg Tl/L in 8M HCl).[8] Therefore, recovering the Pb from Sr or Pb resin with HCl can lead to the formation of a thallium chloride precipitate if the thallium target material is not completely removed in the load and rinsing steps.
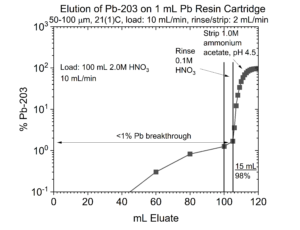
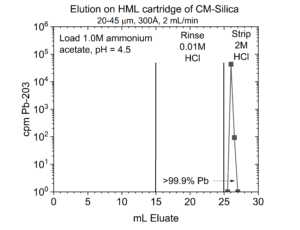
An alternative is to recover the Pb from the Pb resin in 1M ammonium acetate (Figure 3). Thallous acetate is very soluble (~100 g/L), and recovery of the Pb in 1M ammonium acetate buffer facilitates further purification of the Pb using a column of weak acid (CM) silica.
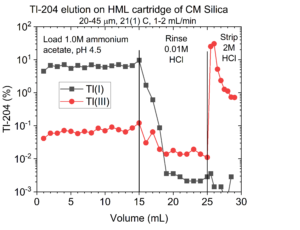
On the CM silica, the Pb is retained from 0.1-2.0M ammonium acetate, pH 4.5, while Tl(I) passes through the column (Figure 4).
To achieve the additional Tl decontamination it is important to reduce thallium to Tl(I) using ascorbic acid, as Tl(III) will co-elute with the Pb on the CM-Silica (Figure 5). The can Pb be recovered from the CM silica in 0.25 – 8.0M HCl.
In all applications of Sr or Pb resin for the production of medical radionuclides, it is important to consider not just the radionuclidic purity of the final product, but also the chemical purity. Sr and Pb resin contain a crown ether dissolved in 1-octanol and 2-decanol, respectively. A small amount of the alcohol will leach off of the resin and should be removed using a polymeric (Prefilter Resin) or inorganic adsorbant (tC18-silica) or subsequent separation of Pb on column of CM-silica or covalently bound ion exchange resin.
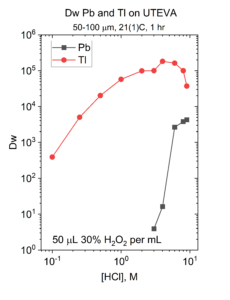
Once 201Pb is purified using chemistry such as the Pb Resin/CM-silica procedure described above, its 201Tl daughter can be milked periodically. The 201Pb/201Tl separation can be achieved by oxidizing thallium to Tl(III) by adding a small volume of 30% H2O2 to the 201Pb in 2M HCl. Tl(III) is strongly retained on UTEVA resin from HCl, while Pb is rejected below ~3-4M HCl (Figure 6).
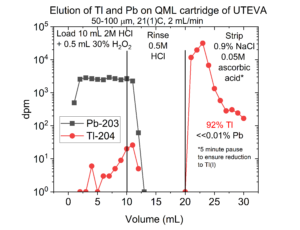
The separation of Tl(III) from Pb on a QML cartridge of UTEVA resin is presented in Figure 7 using 203Pb and 204Tl surrogates. The Pb is quantitatively recovered in the load and 1-2 mL of 0.5M HCl rinse. The Tl is recovered by reducing to Tl(I) with ascorbic acid and eluting in normal saline (0.9% NaCl).
References:
[1] Turkanyi, F.; Ditoi, F.; Hermanne, A.; Takacs, S.; Adam-Rebeles, R.; Walravens, N.; Cichelli, O.; Ignatyuk, A.V.; Investigation of activation cross-sections of proton induced nuclear reactions on natTl up to 42 MeV: review, new data and evaluation, Applied Radiation and Isotopes, 74, 109-122 (2013).
[2] Saini, S.; Bartels, J.L.; Appiah, J-P.K.; Rider, J.H.; Baumhover, N.; Schultz, M.K.; Lapi, S.E.; Optimized methods for the Production of High-Purity 203Pb Using Electroplated Thallium Targets, Journal of Nuclear Medicine, 64(11), 1791-1797 (2023).
[3] Li, M., Zhang, X., Quinn, T., Lee, D., Liu, D., Kunkel, F., Zimmerman, B., McAlister, D.R., Olewein, K., Menda, Y., Mirzadeh, S., Copping, R., Johnson, F.L., Shultz, M.K., 2017. “Automated cassette-based production of high specific activity [203/212Pb]peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer,” Applied Radiation and Isotopes, 127, 52-60.
[4] Atcher, R.W.; Friedman, A.M.; Hines, J.J.; An improved generator for the production of 212Pb and 212Bi from 224Ra, Int J Appl Instrum A, 39(4), 283-286 (1988).
[5] Horwitz, E.P.; Chiarizia, R.; Dietz, M.L.; A Novel Strontium-Selective Extraction Chromatographic Resin, Solv. Extr. Ion Exchange, 10(2), 313-336 (1992).
[6] Horwitz, E.P.; Dietz, M.L.; Rhoads, S.; Felinto, C.; Gale, N.; Houghton, J.; A Lead-Selective Extraction Chromatographic Resin and Its Application to the Isolation of Lead from Geologic Samples, Anal. Chim. Acta, 292(3), 263-273 (1994).
[7] “The hydrolysis of cations,” Charles F. Baes Jr. and Robert E. Mesmer, John Wiley and Sons, New York, 1976, pp. 328 – 335.
[8] Handbook of Chemistry and Physics, Editor-in-Chief David R. Lide, 83rd Edition, CRC Press, New York, 8-117 to 8-120 2002-2003.